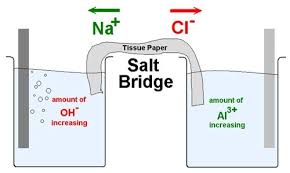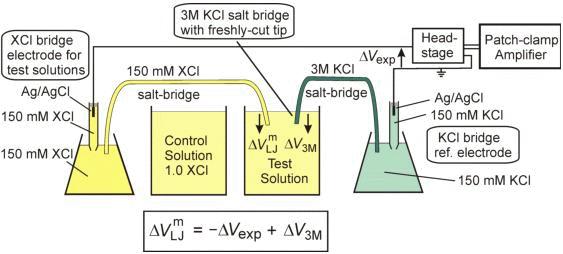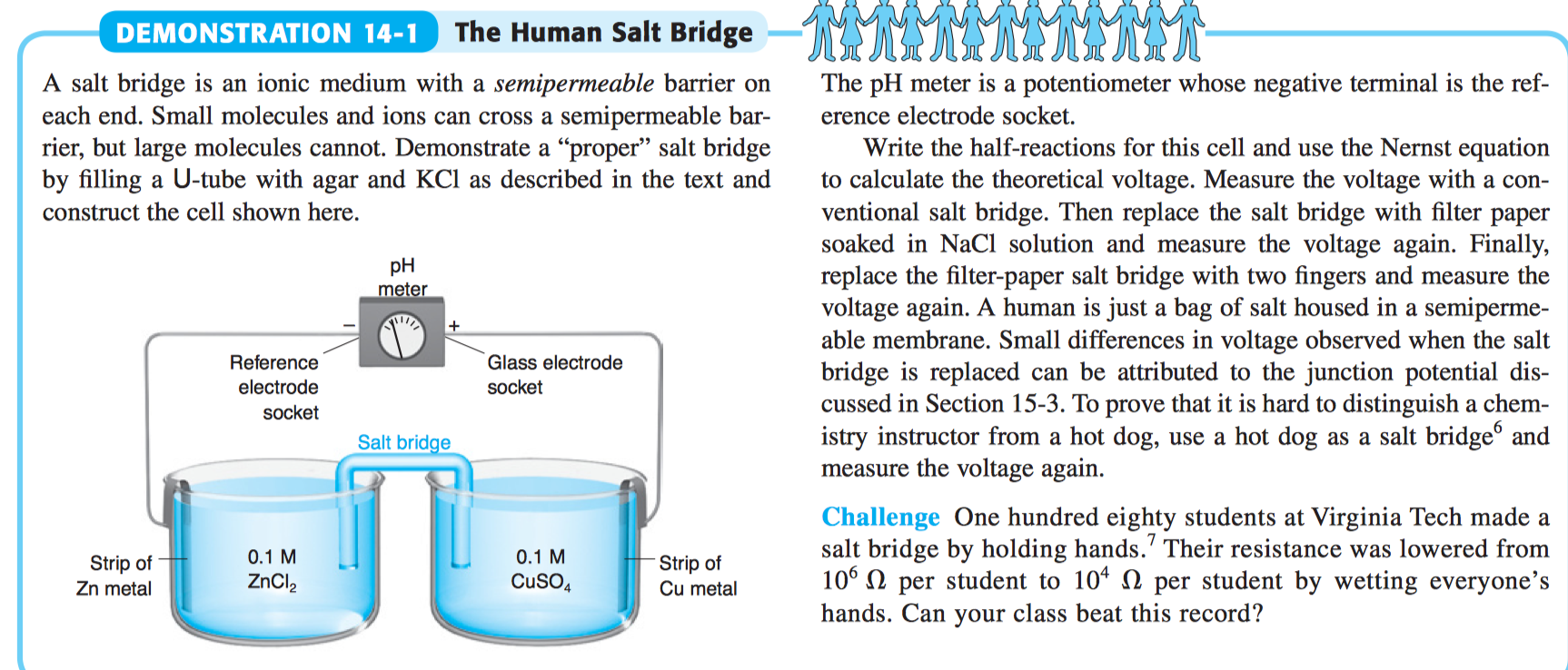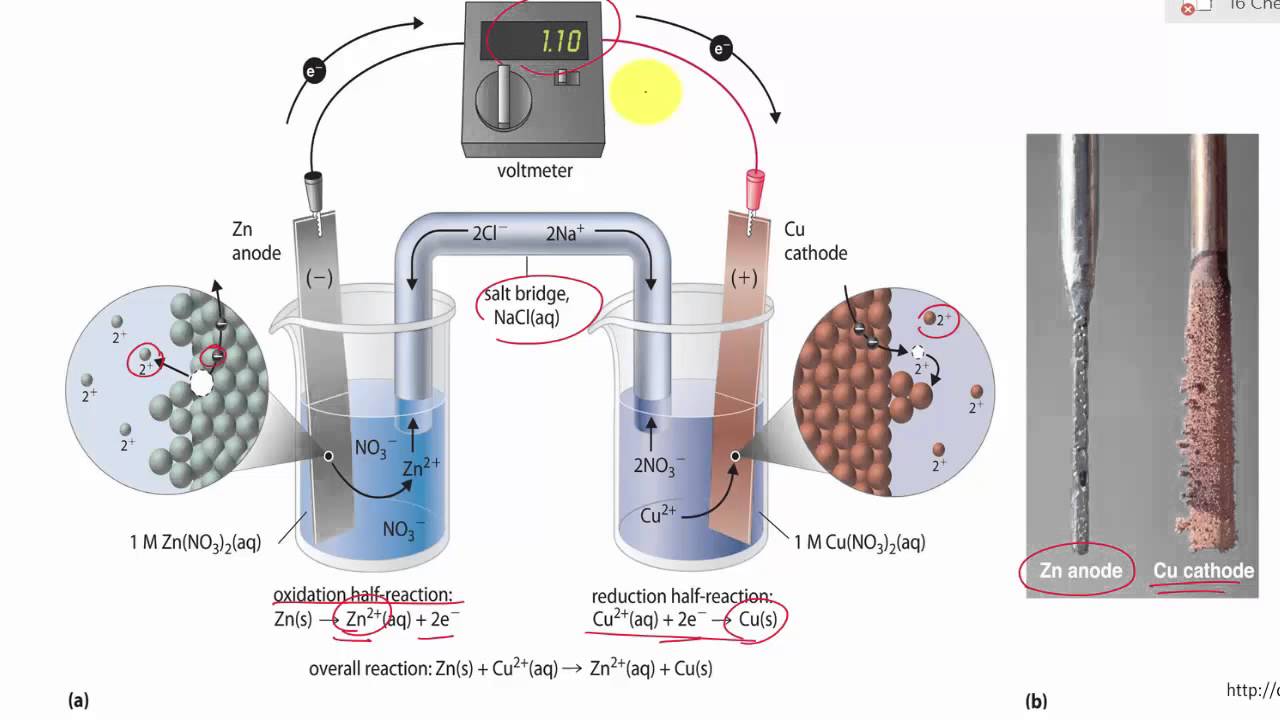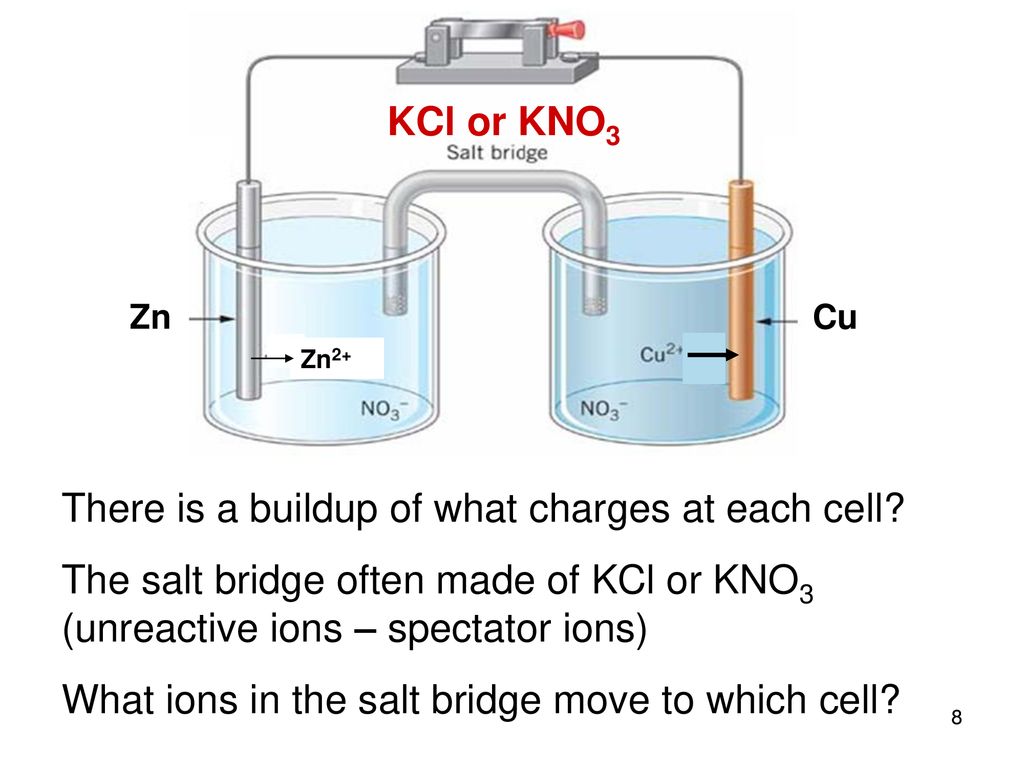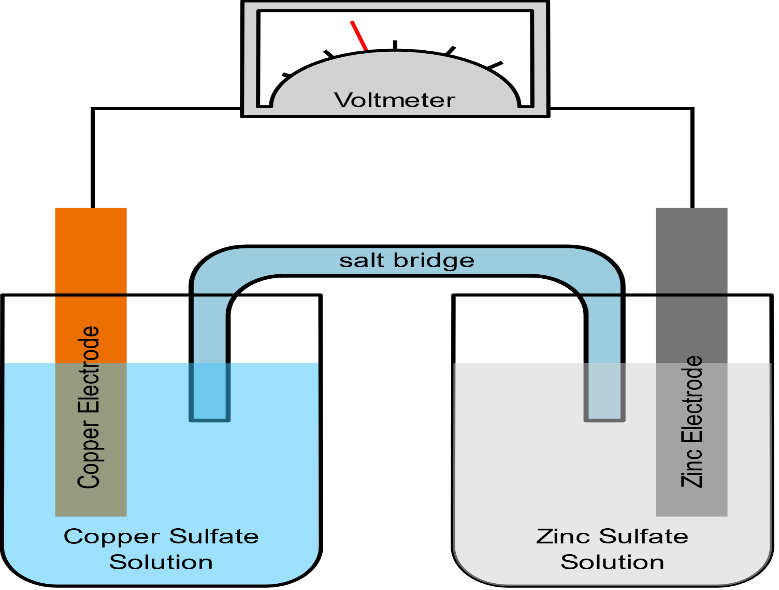
The function(s) of salt bridge in a cell is\/areA. It maintains standard electrode potential of cell constant which depends on several factors.B. It completes the electrical circuit.C. It departs both the solutions

Statement I: KCl, NaCl, NH4Cl, etc., cannot be used in the salt bridge of a cell containing silver.Statement II : A salt bridge contains concentrated solution of an inert electrolyte like KCl,
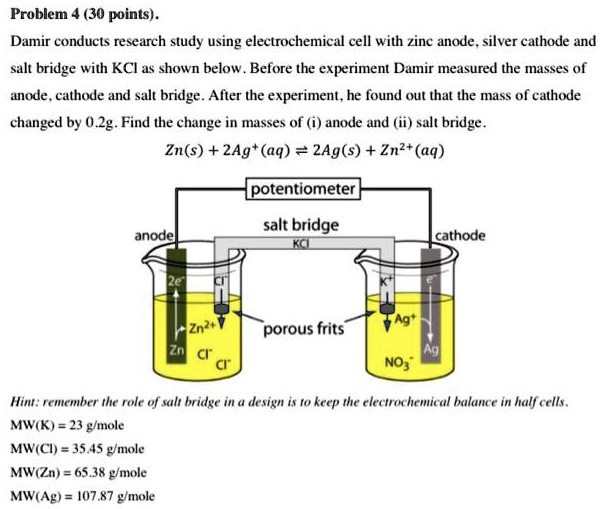
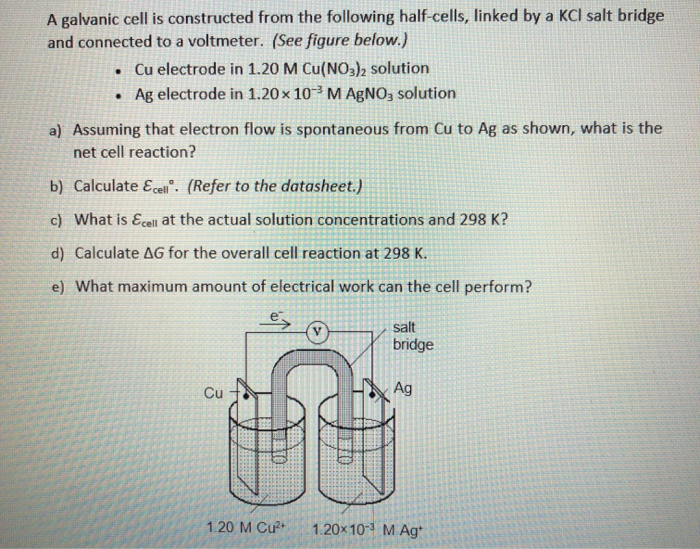
SOLVED: Problem 4 (30 points) . Damir conducts research study using electrochemical cell with zine anode silver cathode und salt bridge with KCl as shown below. Before the experiment Damir measured the

A reaction: 12H2(g) + AgCl(s) H^⊕(aq) + Cl^ (aq) + Ag(s) occurs in a galvanic cell. The structure of the cell will be: